One of the greatest joys for a synthetic organometallic chemist is to see the molecule they spent weeks, months or years synthesizing rendered in 3D by way of the science (and magic!) of single crystal X-ray diffraction. Below are a handful of the Thompson Lab's favorite molecular curios captured by X-ray diffraction. The art & beauty of synthesis is on display with this growing collection.
|
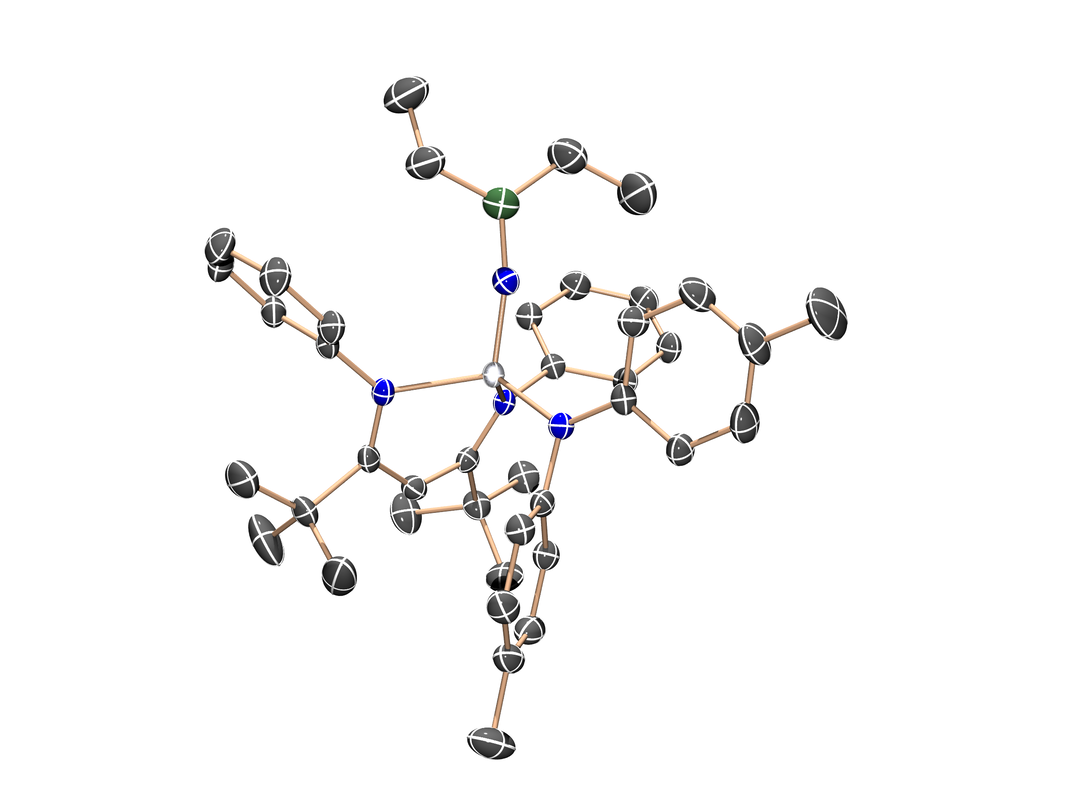
Titanium borylimido
(J. Am. Chem. Soc. 2014, 136, 8197-8200) In an effort to synthesize the first terminal titanium nitride (Ti≡N), the parent imido (Ti=NH) was reacted with sodium triethylborohydride. What formed (cleanly!) instead was this rare instance of a borylimido. The Ti=N bond was a suitable platform for other exotic substituents including dimethylaluminyl and diisopropylphosphino. The Tebbe Reagent
(Organometallics 2014, 33, 429-432) The Tebbe Reagent is a famous titanium methylidene capable of carbonyl methylidenations too difficult for a Wittig reagent. Despite being reported in 1978 by Fred Tebbe at DuPont its crystal structure wasn't reported until nearly 40 years later. |
Molybdenum Metallatetrahedrane
(Organometallics 2019, 38, 4054-4059) While attempting to isolate a metallacyclobutadiene intermediate formed during catalytic alkyne metathesis reactions, this isomeric tetrahedrane arose. It's observation as an isolable but dynamic species warranted further investigation which led to a follow up paper (J. Am. Chem. Soc. 2021, 143, 9026-9039)! |
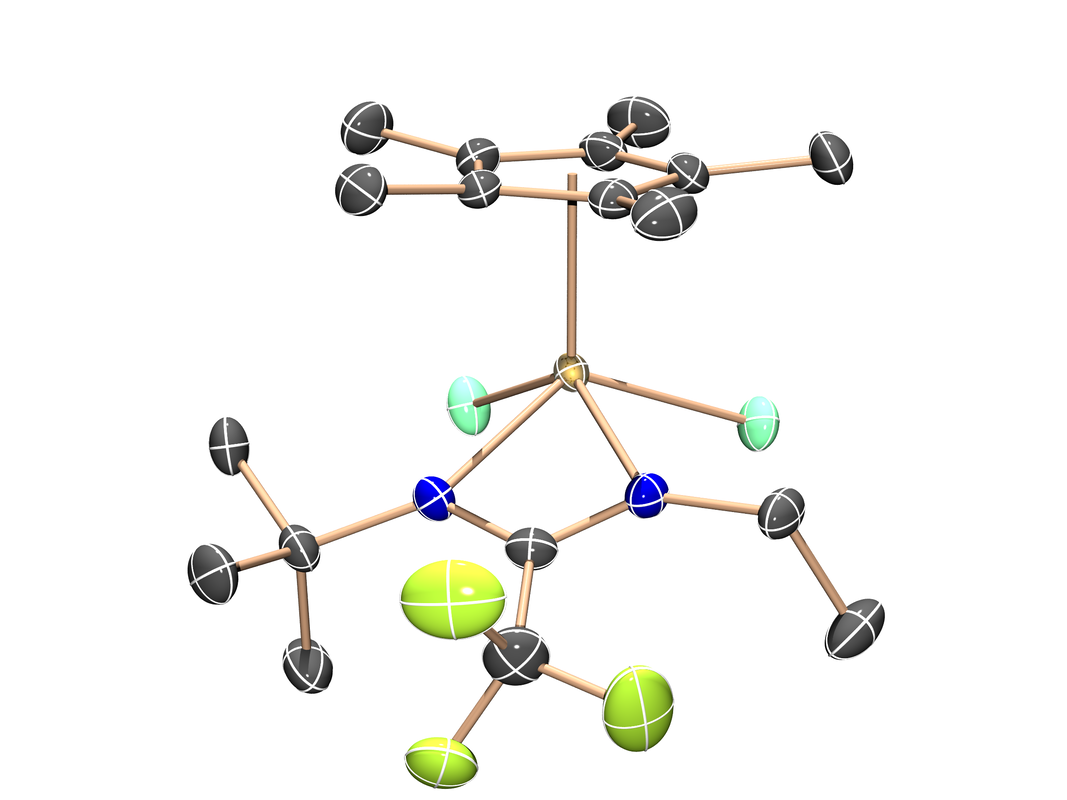
Hafnium Olefin Polymerization Catalyst
(Organometallics, 2019, 38, 213-217) The cyclopentadienyl-amidinate mixed ligand set was a workhorse for group IV catalyzed, α-olefin polymerization reactions. The lower activity of hafnium relative to zirconium, however, limited its practical utility. Substitution of the amidinate distal group from methyl to trifluoromethyl, a 6-8 fold increase in rate of polymerization occurred. |
THOMPSON LAB @ UNIVERSITY OF IDAHO
MOSCOW ID 83844
MOSCOW ID 83844
Established 2023